Lactic acid fermentation
Lactic acid fermentation is a metabolic process by which glucose and other six-carbon sugars (also, disaccharides of six-carbon sugars, e.g. sucrose or lactose) are converted into cellular energy and the metabolite lactate, which is lactic acid in solution. It is an anaerobic fermentation reaction that occurs in some bacteria and animal cells, such as muscle cells.[1][2][3]
If oxygen is present in the cell, many organisms will bypass fermentation and undergo cellular respiration; however, facultative anaerobic organisms will both ferment and undergo respiration in the presence of oxygen.[3] Sometimes even when oxygen is present and aerobic metabolism is happening in the mitochondria, if pyruvate is building up faster than it can be metabolized, the fermentation will happen anyway.
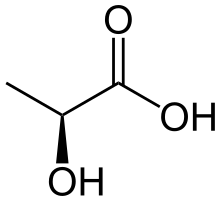
Lactate dehydrogenase catalyzes the interconversion of pyruvate and lactate with concomitant interconversion of NADH and NAD+.
In homolactic fermentation, one molecule of glucose is ultimately converted to two molecules of lactic acid. Heterolactic fermentation, in contrast, yields carbon dioxide and ethanol in addition to lactic acid, in a process called the phosphoketolase pathway.[1]
Contents
History[edit]
Several chemists discovered during the 19th century some fundamental concepts of the domain of organic chemistry. One of them for example was the French chemist Joseph Louis Gay-Lussac, who was especially interested in fermentation processes, and he passed this fascination to one of his best students, Justus von Liebig. With a difference of some years, each of them described, together with colleague, the chemical structure of the lactic acid molecule as we know it today. They had a purely chemical understanding of the fermentation process, which means that you can’t see it using a microscope, and that it can only be optimized by chemical catalyzers. It was then in 1857 when the French chemist Louis Pasteur first described the lactic acid as the product of a microbial fermentation. During this time, he worked at the university of Lille, where a local distillery asked him for advice concerning some fermentation problems. Per chance and with the badly equipped laboratory he had at that time, he was able to discover that in this distillery, two fermentations were taking place, a lactic acid one and an alcoholic one, both induced by some microorganisms. He then continued the research on these discoveries in Paris, where he also published his theories that presented a stable contradiction to the purely chemical version represented by Liebig and his followers. Even though Pasteur described some concepts that are still accepted nowadays, Liebig refused to accept them until his death in 1873. But even Pasteur himself wrote that he was “driven” to a completely new understanding of this chemical phenomenon. Even if Pasteur didn’t find every detail of this process, he still discovered the main mechanism of how the microbial lactic acid fermentation works. He was for example the first to describe fermentation as a “form of life without air."[4][5]
Even if this chemical process hasn’t been properly described before Pasteur’s work, people had been using microbial lactic acid fermentation for food production much earlier. Chemical analysis of archeological finds showed that milk fermentation uses predate the historical period, its first applications were probably a part of the Neolithic Revolution. Since milk naturally contains lactic acid bacteria, the discovery of the fermentation process was quite evident, since it happens spontaneously at an adequate temperature. The problem of these first farmers was that fresh milk is nearly not digestible by an adult, so they had an interest to discover this mechanism: In fact, lactic acid bacteria contain the needed enzymes to digest lactose, and their populations multiply strongly during the fermentation. Therefore, even short-fermented milk contains enough enzymes to digest the lactose molecules, once the milk is in the human body, what allowed also adults to consume it. Even safer was a longer fermentation, which was practiced for cheesemaking. This process was discovered a very long time ago too, what is proofed by recipes for cheese production on Cuneiform scripts, the first written documents that exist, and a bit later in Babylonian and Egyptian texts. What's interesting is the theory of the competitive advantage of fermented milk products. The idea of this theory is that the women of these first settled farmer clans could shorten the time between two children thanks to the additional lactose uptake from milk consumption. This factor may have given them an important advantage to out-compete the hunter-gatherer societies.[6]
With the increasing consumption of milk products these societies developed a lactase persistence by epigenetic inheritance, which means that the milk-digesting enzyme lactase was present in their body during the whole lifetime, so they could drink unfermented milk as adults too. This early habituation to lactose consumption in the first settler societies can still be observed today on regional differences of this mutation’s concentration. It’s estimated that about 65% of world population still lacks it.[7] Since these first societies came from regions around eastern Turkey to central Europe, the gene appears more frequently there and in North America, as it was settled by Europeans. On the contrary, lactose intolerance is much more present in Asian countries.
Milk products and their fermentation have had an important influence on some cultures’ development. This is the case in Mongolia, where people often practice a pastoral form of agriculture. The milk that they produce and consume in these cultures is mainly mare milk and has a long tradition. But not every part or product of the fresh milk has the same meaning. For instance, the fattier part on the top, the “deež”, is seen as the most valuable part and is therefore often used to honor guests. Very important with often a traditional meaning as well are fermentation products of mare milk, like for example the slightly-alcoholic yogurt kumis. Consumption of these peaks during cultural festivities such as the Mongolian lunar new year (in spring). The time of this celebration is called the “white month”, which indicates that milk products (called “white food” together with starchy vegetables, in comparison to meat products, called “black food”) are a central part of this tradition. The purpose of these festivities is to “close” the past year – clean the house or the yurt, honor the animals for having provided their food, and prepare everything for the coming summer season – to be ready to “open” the new year. Consuming white food in this festive context is a way to connect to the past and to a national identity, which is the great Mongolian empirepersonified by Genghis Khan. During the time of this empire, the fermented mare milk was the drink to honor and thank warriors and leading persons, it was not meant for everybody. Even though it can became a drink for normal people, it has kept its honorable meaning. Like many other traditions, this one feels the influence of the globalizing industry. Other products, coming mainly from China and western countries, like industrial yogurt, tend to replace it more and more, mainly in urban areas. However, in rural and poorer regions it’s still of great importance.[8]
Biochemistry[edit]
Homofermentative process[edit]
Overall, the homofermentative lactic acid fermentation converts a six-carbon sugar molecule to two lactic acid molecules, storing the released energy into two ATP molecules. The following equation describes this net result:
Heterofermentative process[edit]
Heterofermentative bacteria produce one mole of lactate from one mole of glucose as well as CO2 and acetic acid or ethanol. Examples include Leuconostoc mesenteroides, Lactobacillus bifermentous, and Leconostoc lactis.
Applications[edit]
Lactic acid fermentation is used in many areas of the world to produce foods that cannot be produced through other methods.[9][10] The most commercially important genus of lactic acid-fermenting bacteria is Lactobacillus, though other bacteria and even yeast are sometimes used.[9] Two of the most common applications of lactic acid fermentation are in the production of yogurt and sauerkraut.
Pickle[edit]
A product prepared by lactic acid bacteria (LAB) fermentation of sugars present in the pieces of fruits and vegetables. The prepared product is rich in lactic acid, and only the beneficial bacteria that can tolerate lactic acid pH survive. It not only assures good quality of nutrients, but it is also a good source of probiotics.
Kimchi[edit]
Sauerkraut[edit]
Lactic acid fermentation is also used in the production of sauerkraut. The main type of bacteria used in the production of sauerkraut is of the genus Leuconostoc.[1][12]
As in yogurt, when the acidity rises due to lactic acid-fermenting organisms, many other pathogenic microorganisms are killed. The bacteria produce lactic acid, as well as simple alcohols and other hydrocarbons. These may then combine to form esters, contributing to the unique flavor of sauerkraut.[1]
Sour beer[edit]
Lactic acid is a component in the production of sour beers, including Lambics and Berliner Weisses.[13]
Yogurt[edit]
The main method of producing yogurt is through the lactic acid fermentation of milk with harmless bacteria.[9][14] The primary bacteria used are typically Lactobacillus bulgaricus and Streptococcus thermophilus, and United States as well as European law requires all yogurts to contain these two cultures (though others may be added as probiotic cultures).[14] These bacteria produce lactic acid in the milk culture, decreasing its pH and causing it to congeal. The bacteria also produce compounds that give yogurt its distinctive flavor. An additional effect of the lowered pH is the incompatibility of the acidic environment with many other types of harmful bacteria.[9][14]
For a probiotic yogurt, additional types of bacteria such as Lactobacillus acidophilus are also added to the culture.[14]
Physiological[edit]
Lactobacillus fermentation and accompanying production of acid provides a protective vaginal microbiome that protects against the proliferation of pathogenic organisms
are no longer able to grow. Leuconostocs and lactic streptococci generally lower the pH to about 4.0-4.5 and some of the lactobacilli and pediococci to about 3.5 before inhibiting their own growth. In addition to producing lactic acid, the lactobacilli also have the ability to produce hydrogen peroxide through oxidation of reduced nicotinamide adenine dinucleotide (NADH) by flavin nucleotides which react rapidly with gaseous oxygen (Hurst and Collins-Thompson, 1979). Flavoproteins such as glucose oxidase also generate hydrogen peroxide and produce an antibiotic effect on other organisms that might cause food spoilage. The lactobacilli themselves are relatively resistant to hydrogen peroxide. For example, Wheater et al. (1952) showed that Lactobaeillus lactis required a concentration of 125 ~tg hydrogen peroxide/ ml to inhibit an inoculum of 104cells/ml while Staphylococcus aureus was inhibited by a concentration of 4 ~tg hydrogen perioxide/ml even though the latter contains catalase which is lacking in Lb. lactis. Streptococcus lactis produces the polypeptide antibiotic nisin active against gram-positive organisms including S. cremoris which, in turn, produces an antibiotic "diplococcin" active against gram-positive organisms including S. lactis. Thus, these two organisms compete in the fermentation of milk products while inhibiting growth of other gram-positive bacteria (Hirsch, 1952). Carbon dioxide produced by heterofermentative lactobacilli also has a preservative effect in foods resulting among others, from its flushing action leading to anaerobiosis if the substrate is properly protected.
Lactic acid bacteria perform an essential role in the preservation and production of wholesome foods. Generally the lactic acid fermentations are low-cost and often little or no heat is required in their preparation. Thus, they are fuelefficient. Lactic acid fermented foods have an important role in feeding the world's population on every continent today. As world population rises, lactic acid fermentation is expected to become even more important in preserving fresh vegetables, fruits, cereals and legumes for feeding humanity. INTRODUCTION Lactic acid bacteria perform an essential role in the preservation and production of wholesome foods ranging from fermented fresh vegetables such as cabbage (sauerkraut/Korean kimchi) and cucumbers (pickles) to fermented cereal yogurt (Nigerian ogi/Kenyan uji), to sour-dough bread and breadlike products without the use of wheat or rye flours (Indian idli/Philippine puto), to fermented milks (yogurts/cheeses), to fermented milk/wheat mixtures (Egyptian kishk/ Greek trahanas), to protein-rich, vegetable protein meat substitutes (Indonesian tempe), to amino/peptide meat flavoured sauces and pastes produced by fermentation of cereals/legumes (Japanese miso/Chinese soy sauce), to fermented cereal/fish/shrimp mixtures (Philippine balao/balao; Philippine burong dalag), to fermented meats (European salami, etc.). Both the homofermentative and the heterofermentative lactic acid bacteria are generally fastidious on artificial media but they grow readily in most food substrates and lower the pH rapidly to a point where other competing organisms Brining and lactic acid fermentation continue to be highly desirable methods of processing and preserving vegetables because they are low-cost, have low energy requirements for both processing and preparing foods for consumption and they yield highly acceptable and diversified flavours for humans. Depending upon the salt concentration salting directs the subsequent course of the fermentation limiting the amount of pectinolytic and proteolytic hydrolysis that occurs thus controlling softening and preventing putrefaction. Lactic acid fermentations have some other distinct advantages in that the foods become resistant to microbial spoilage and to development of toxins. Acid foods are less likely to transfer pathogenic microorganisms. Acid fermentations also modify the flavour of the original ingredients and often improve nutritive value. Since canned or frozen foods are unavailable or too expensive for hundreds of millions of the world's economically deprived and hungry, acid fermentation combined with salting remains one of the most practical methods of preservation often enhancing organoleptic and nutritional quality of fresh vegetables, cereal gruels and milk-cereal mixtures. Lactic acid fermentation is utilized as a major method of processing and preserving vegetables, cereals and legumes throughout the world and particularly in the developing world. It is likely to become even more important as world population will have increased to about 6 billion by the year 2000 and to an expected 8-12 billion in the 21st century. This paper will review some of the vegetable/cereal grain/legume foods involv-
are no longer able to grow. Leuconostocs and lactic streptococci generally lower the pH to about 4.0-4.5 and some of the lactobacilli and pediococci to about 3.5 before inhibiting their own growth. In addition to producing lactic acid, the lactobacilli also have the ability to produce hydrogen peroxide through oxidation of reduced nicotinamide adenine dinucleotide (NADH) by flavin nucleotides which react rapidly with gaseous oxygen (Hurst and Collins-Thompson, 1979). Flavoproteins such as glucose oxidase also generate hydrogen peroxide and produce an antibiotic effect on other organisms that might cause food spoilage. The lactobacilli themselves are relatively resistant to hydrogen peroxide. For example, Wheater et al. (1952) showed that Lactobaeillus lactis required a concentration of 125 ~tg hydrogen peroxide/ ml to inhibit an inoculum of 104cells/ml while Staphylococcus aureus was inhibited by a concentration of 4 ~tg hydrogen perioxide/ml even though the latter contains catalase which is lacking in Lb. lactis. Streptococcus lactis produces the polypeptide antibiotic nisin active against gram-positive organisms including S. cremoris which, in turn, produces an antibiotic "diplococcin" active against gram-positive organisms including S. lactis. Thus, these two organisms compete in the fermentation of milk products while inhibiting growth of other gram-positive bacteria (Hirsch, 1952). Carbon dioxide produced by heterofermentative lactobacilli also has a preservative effect in foods resulting among others, from its flushing action leading to anaerobiosis if the substrate is properly protected.
Lactic acid bacteria perform an essential role in the preservation and production of wholesome foods. Generally the lactic acid fermentations are low-cost and often little or no heat is required in their preparation. Thus, they are fuelefficient. Lactic acid fermented foods have an important role in feeding the world's population on every continent today. As world population rises, lactic acid fermentation is expected to become even more important in preserving fresh vegetables, fruits, cereals and legumes for feeding humanity. INTRODUCTION Lactic acid bacteria perform an essential role in the preservation and production of wholesome foods ranging from fermented fresh vegetables such as cabbage (sauerkraut/Korean kimchi) and cucumbers (pickles) to fermented cereal yogurt (Nigerian ogi/Kenyan uji), to sour-dough bread and breadlike products without the use of wheat or rye flours (Indian idli/Philippine puto), to fermented milks (yogurts/cheeses), to fermented milk/wheat mixtures (Egyptian kishk/ Greek trahanas), to protein-rich, vegetable protein meat substitutes (Indonesian tempe), to amino/peptide meat flavoured sauces and pastes produced by fermentation of cereals/legumes (Japanese miso/Chinese soy sauce), to fermented cereal/fish/shrimp mixtures (Philippine balao/balao; Philippine burong dalag), to fermented meats (European salami, etc.). Both the homofermentative and the heterofermentative lactic acid bacteria are generally fastidious on artificial media but they grow readily in most food substrates and lower the pH rapidly to a point where other competing organisms Brining and lactic acid fermentation continue to be highly desirable methods of processing and preserving vegetables because they are low-cost, have low energy requirements for both processing and preparing foods for consumption and they yield highly acceptable and diversified flavours for humans. Depending upon the salt concentration salting directs the subsequent course of the fermentation limiting the amount of pectinolytic and proteolytic hydrolysis that occurs thus controlling softening and preventing putrefaction. Lactic acid fermentations have some other distinct advantages in that the foods become resistant to microbial spoilage and to development of toxins. Acid foods are less likely to transfer pathogenic microorganisms. Acid fermentations also modify the flavour of the original ingredients and often improve nutritive value. Since canned or frozen foods are unavailable or too expensive for hundreds of millions of the world's economically deprived and hungry, acid fermentation combined with salting remains one of the most practical methods of preservation often enhancing organoleptic and nutritional quality of fresh vegetables, cereal gruels and milk-cereal mixtures. Lactic acid fermentation is utilized as a major method of processing and preserving vegetables, cereals and legumes throughout the world and particularly in the developing world. It is likely to become even more important as world population will have increased to about 6 billion by the year 2000 and to an expected 8-12 billion in the 21st century. This paper will review some of the vegetable/cereal grain/legume foods involv-



No comments:
Post a Comment