Ribosome
| Cell biology | |
|---|---|
| The animal cell | |
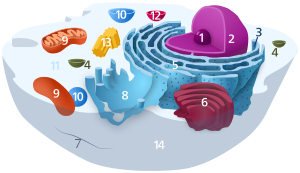
Components of a typical animal cell:
|
The ribosome (/ˈraɪbəˌsoʊm, -boʊ-/[1]) is a complex molecular machine, found within all living cells, that serves as the site of biological protein synthesis (translation). Ribosomes link amino acids together in the order specified by messenger RNA (mRNA) molecules. Ribosomes consist of two major components: the small ribosomal subunits, which reads the RNA, and the large subunits, which joins amino acids to form a polypeptidechain. Each subunit is composed of one or more ribosomal RNA (rRNA) molecules and a variety of ribosomal proteins (r-protein or rProtein[2][3][4]). The ribosomes and associated molecules are also known as the translational apparatus.
Contents
[hide]Overview[edit]
The sequence of DNA, which encodes the sequence of the amino acids in a protein, is copied into a messenger RNA chain. It may be copied many times into RNA chains. Ribosomes can bind to a messenger RNA chain and use its sequence for determining the correct sequence of amino acids. Amino acids are selected, collected, and carried to the ribosome by transfer RNA (tRNA) molecules, which enter one part of the ribosome and bind to the messenger RNA chain. It is during this binding that the correct translation of nucleic acid sequence to amino acid sequence occurs. For each coding triplet in the messenger RNA there is a distinct transfer RNA that matches and which carries the correct amino acid for that coding triplet. The attached amino acids are then linked together by another part of the ribosome. Once the protein is produced, it can then fold to produce a specific functional three-dimensional structure although during synthesis some proteins start folding into their correct form.
A ribosome is made from complexes of RNAs and proteins and is therefore a ribonucleoprotein. Each ribosome is divided into two subunits:
- a smaller subunit which binds to a larger subunit and the mRNA pattern, and
- a larger subunit which binds to the tRNA, the amino acids, and the smaller subunit.
When a ribosome finishes reading an mRNA molecule, these two subunits split apart. Ribosomes are ribozymes, because the catalytic peptidyl transferase activity that links amino acids together is performed by the ribosomal RNA. Ribosomes are often associated with the intracellular membranes that make up the rough endoplasmic reticulum.
Ribosomes from bacteria, archaea and eukaryotes in the three-domain system, resemble each other to a remarkable degree, evidence of a common origin. They differ in their size, sequence, structure, and the ratio of protein to RNA. The differences in structure allow some antibiotics to kill bacteria by inhibiting their ribosomes, while leaving human ribosomes unaffected. In bacteria and archaea, more than one ribosome may move along a single mRNA chain at one time, each "reading" its sequence and producing a corresponding protein molecule.
The mitochondrial ribosomes of eukaryotic cells, are produced from mitochondrial genes, and functionally resemble many features of those in bacteria, reflecting the likely evolutionary origin of mitochondria.[5][6]
Discovery[edit]
Ribosomes were first observed in the mid-1950s by Romanian-American cell biologist George Emil Palade, using an electron microscope, as dense particles or granules.[7] The term "ribosome" was proposed by scientist Richard B. Roberts in the end of 1950s:
Albert Claude, Christian de Duve, and George Emil Palade were jointly awarded the Nobel Prize in Physiology or Medicine, in 1974, for the discovery of the ribosome.[9] The Nobel Prize in Chemistry 2009 was awarded to Venkatraman Ramakrishnan, Thomas A. Steitzand Ada E. Yonath for determining the detailed structure and mechanism of the ribosome.[10]
Structure[edit]
The ribosome is a highly complex cellular machine. It is largely made up of specialized RNA known as ribosomal RNA (rRNA) as well as dozens of distinct proteins (the exact number varies slightly between species). The ribosomal proteins and rRNAs are arranged into two distinct ribosomal pieces of different size, known generally as the large and small subunit of the ribosome. Ribosomes consist of two subunits that fit together (Figure 2) and work as one to translate the mRNA into a polypeptide chain during protein synthesis (Figure 1). Because they are formed from two subunits of non-equal size, they are slightly longer in the axis than in diameter.
Prokaryotic ribosomes are around 20 nm (200 Å) in diameter and are composed of 65% rRNA and 35% ribosomal proteins.[11] Eukaryotic ribosomes are between 25 and 30 nm (250–300 Å) in diameter with an rRNA-to-protein ratio that is close to 1.[12] Crystallographic work [13] has shown that there are no ribosomal proteins close to the reaction site for polypeptide synthesis. This suggests that the protein components of ribosomes do not directly participate in peptide bond formation catalysis, but rather that these proteins act as a scaffold that may enhance the ability of rRNA to synthesize protein (See: Ribozyme).
The ribosomal subunits of prokaryotes and eukaryotes are quite similar.[15]
The unit of measurement used to describe the ribosomal subunits and the rRNA fragments is the Svedberg unit, a measure of the rate of sedimentation in centrifugation rather than size. This accounts for why fragment names do not add up: for example, prokaryotic 70S ribosomes are made of 50S and 30S subunits.
Prokaryotes have 70S ribosomes, each consisting of a small (30S) and a large (50S) subunit. Their small subunit has a 16S RNA subunit (consisting of 1540 nucleotides) bound to 21 proteins. The large subunit is composed of a 5S RNA subunit (120 nucleotides), a 23S RNA subunit (2900 nucleotides) and 31 proteins.[15]
| ribosome | subunit | rRNAs | r-proteins |
|---|---|---|---|
| 70S | 50S | 23S (2904 nt) | 31 |
| 5S (120 nt) | |||
| 30S | 16S (1542 nt) | 21 |
Affinity label for the tRNA binding sites on the E. coli ribosome allowed the identification of A and P site proteins most likely associated with the peptidyltransferase activity; labelled proteins are L27, L14, L15, L16, L2; at least L27 is located at the donor site, as shown by E. Collatz and A.P. Czernilofsky.[17][18] Additional research has demonstrated that the S1 and S21 proteins, in association with the 3'-end of 16S ribosomal RNA, are involved in the initiation of translation.[19]
Eukaryotes have 80S ribosomes, each consisting of a small (40S) and large (60S) subunit. Their 40S subunit has an 18S RNA (1900 nucleotides) and 33 proteins.[20][21] The large subunit is composed of a 5S RNA(120 nucleotides), 28S RNA (4700 nucleotides), a 5.8S RNA (160 nucleotides) subunits and 46 proteins.[15][20][22]
| ribosome | subunit | rRNAs | r-proteins |
|---|---|---|---|
| 80S | 60S | 28S (4718 nt) | 49 |
| 5.8S (160 nt) | |||
| 5S (120 nt) | |||
| 40S | 18S (1874 nt) | 33 |
During 1977, Czernilofsky published research that used affinity labeling to identify tRNA-binding sites on rat liver ribosomes. Several proteins, including L32/33, L36, L21, L23, L28/29 and L13 were implicated as being at or near the peptidyl transferase center.[24]
The ribosomes found in chloroplasts and mitochondria of eukaryotes also consist of large and small subunits bound together with proteins into one 70S particle.[15] These organelles are believed to be descendants of bacteria (see Endosymbiotic theory) and, as such, their ribosomes are similar to those of bacteria.[15]
The various ribosomes share a core structure, which is quite similar despite the large differences in size. Much of the RNA is highly organized into various tertiary structural motifs, for example pseudoknots that exhibit coaxial stacking. The extra RNA in the larger ribosomes is in several long continuous insertions, such that they form loops out of the core structure without disrupting or changing it.[15] All of the catalytic activity of the ribosome is carried out by the RNA; the proteins reside on the surface and seem to stabilize the structure.[15]
The differences between the bacterial and eukaryotic ribosomes are exploited by pharmaceutical chemists to create antibiotics that can destroy a bacterial infection without harming the cells of the infected person. Due to the differences in their structures, the bacterial 70S ribosomes are vulnerable to these antibiotics while the eukaryotic 80S ribosomes are not.[25] Even though mitochondria possess ribosomes similar to the bacterial ones, mitochondria are not affected by these antibiotics because they are surrounded by a double membrane that does not easily admit these antibiotics into the organelle.[26]
High-resolution structure[edit]
The general molecular structure of the ribosome has been known since the early 1970s. In the early 2000s, the structure has been achieved at high resolutions, of the order of a few Å.
The first papers giving the structure of the ribosome at atomic resolution were published almost simultaneously in late 2000. The 50S (large prokaryotic) subunit was determined from the archaeon Haloarcula marismortui[27] and the bacterium Deinococcus radiodurans,[28] and the structure of the 30S subunit was determined from Thermus thermophilus.[14] These structural studies were awarded the Nobel Prize in Chemistry in 2009. In May 2001 these coordinates were used to reconstruct the entire T. thermophilus 70S particle at 5.5 Å resolution.[29]
Two papers were published in November 2005 with structures of the Escherichia coli 70S ribosome. The structures of a vacant ribosome were determined at 3.5-Å resolution using x-ray crystallography.[30] Then, two weeks later, a structure based on cryo-electron microscopy was published,[31] which depicts the ribosome at 11–15Å resolution in the act of passing a newly synthesized protein strand into the protein-conducting channel.
The first atomic structures of the ribosome complexed with tRNA and mRNA molecules were solved by using X-ray crystallography by two groups independently, at 2.8 Å[32] and at 3.7 Å.[33] These structures allow one to see the details of interactions of the Thermus thermophilus ribosome with mRNA and with tRNAs bound at classical ribosomal sites. Interactions of the ribosome with long mRNAs containing Shine-Dalgarno sequences were visualized soon after that at 4.5- to 5.5-Å resolution.[34]
In 2011, the first complete atomic structure of the eukaryotic 80S ribosome from the yeast Saccharomyces cerevisiae was obtained by crystallography.[20] The model reveals the architecture of eukaryote-specific elements and their interaction with the universally conserved core. At the same time, the complete model of a eukaryotic 40S ribosomal structure in Tetrahymena thermophila was published and described the structure of the 40S subunit, as well as much about the 40S subunit's interaction with eIF1 during translation initiation.[21] Similarly, the eukaryotic 60S subunit structure was also determined from Tetrahymena thermophila in complex with eIF6.[22]
Function[edit]
Ribosomes are organelles that synthesize proteins. Proteins are needed for many cellular functions such as repairing damage or directing chemical processes. Ribosomes can be found floating within the cytoplasm or attached to the endoplasmic reticulum.
Translation[edit]
Ribosomes are the workplaces of protein biosynthesis, the process of translating mRNA into protein. The mRNA comprises a series of codons that dictate to the ribosome the sequence of the amino acids needed to make the protein. Using the mRNA as a template, the ribosome traverses each codon (3 nucleotides) of the mRNA, pairing it with the appropriate amino acid provided by an aminoacyl-tRNA. Aminoacyl-tRNA contains a complementary anticodon on one end and the appropriate amino acid on the other. For fast and accurate recognition of the appropriate tRNA, the ribosome utilizes large conformational changes (conformational proofreading) .[35] The small ribosomal subunit, typically bound to an aminoacyl-tRNA containing the amino acid methionine, binds to an AUG codon on the mRNA and recruits the large ribosomal subunit. The ribosome contains three RNA binding sites, designated A, P and E. The A-site binds an aminoacyl-tRNA;[36] the P-site binds a peptidyl-tRNA (a tRNA bound to the peptide being synthesized); and the E-site (exit) binds a free tRNA before it exits the ribosome. Protein synthesis begins at a start codon AUG near the 5' end of the mRNA. mRNA binds to the P site of the ribosome first. The ribosome is able to identify the start codon by use of the Shine-Dalgarno sequence of the mRNA in prokaryotes and Kozak box in eukaryotes.
Although catalysis of the peptide bond involves the C2 hydroxyl of RNA's P-site adenosine in a proton shuttle mechanism, other steps in protein synthesis (such as translocation) are caused by changes in protein conformations. Since their catalytic core is made of RNA, ribosomes are classified as "ribozymes,"[37] and it is thought that they might be remnants of the RNA world.[38]
In Figure 5, both ribosomal subunits (small and large) assemble at the start codon (towards the 5' end of the RNA). The ribosome uses RNA that matches the current codon (triplet) on the mRNA to append an amino acid to the polypeptide chain. This is done for each triplet on the RNA, while the ribosome moves towards the 3' end of the mRNA. Usually in bacterial cells, several ribosomes are working parallel on a single RNA, forming what is called a polyribosome or polysome.
Addition of translation-independent amino acids[edit]
Presence of a ribosome quality control protein Rqc2 is associated with mRNA-independent protein elongation.[39][40] This elongation is a result of ribosomal addition (via tRNAs brought by Rqc2) of CAT tails: ribosomes extend the C-terminus of a stalled protein with random, translation-independent sequences of alanines and threonines.[41][42]
Würzburg University and Max Planck Institute researches, the results of which were published in Cell Reports and The EMBO magazines in September 2016, have shown that ribosomes have the role of being "a quality control point". Professor Utz Fischer from the University of Würzburg has been researching the assembly of proteins called "macromolecular machines" in the cell for years. He describes this assembly process as LEGO blocks: "Think of it as LEGO bricks at the molecular level: One brick is attached to the next until the product is finished. If only one defective or wrong brick is used, the entire building may be compromised as a result."[43][44][45]
Ribosome locations[edit]
Ribosomes are classified as being either "free" or "membrane-bound".
Free and membrane-bound ribosomes differ only in their spatial distribution; they are identical in structure. Whether the ribosome exists in a free or membrane-bound state depends on the presence of an ER-targeting signal sequence on the protein being synthesized, so an individual ribosome might be membrane-bound when it is making one protein, but free in the cytosol when it makes another protein.
Ribosomes are sometimes referred to as organelles, but the use of the term organelle is often restricted to describing sub-cellular components that include a phospholipid membrane, which ribosomes, being entirely particulate, do not. For this reason, ribosomes may sometimes be described as "non-membranous organelles".
Free ribosomes[edit]
Free ribosomes can move about anywhere in the cytosol, but are excluded from the cell nucleus and other organelles. Proteins that are formed from free ribosomes are released into the cytosol and used within the cell. Since the cytosol contains high concentrations of glutathione and is, therefore, a reducing environment, proteins containing disulfide bonds, which are formed from oxidized cysteine residues, cannot be produced within it.
Membrane-bound ribosomes[edit]
When a ribosome begins to synthesize proteins that are needed in some organelles, the ribosome making this protein can become "membrane-bound". In eukaryotic cells this happens in a region of the endoplasmic reticulum (ER) called the "rough ER". The newly produced polypeptide chains are inserted directly into the ER by the ribosome undertaking vectorial synthesis and are then transported to their destinations, through the secretory pathway. Bound ribosomes usually produce proteins that are used within the plasma membrane or are expelled from the cell via exocytosis.[46]
Biogenesis[edit]
In bacterial cells, ribosomes are synthesized in the cytoplasm through the transcription of multiple ribosome gene operons. In eukaryotes, the process takes place both in the cell cytoplasm and in the nucleolus, which is a region within the cell nucleus. The assembly process involves the coordinated function of over 200 proteins in the synthesis and processing of the four rRNAs, as well as assembly of those rRNAs with the ribosomal proteins.
Origin[edit]
The ribosome may have first originated in an RNA world, appearing as a self-replicating complex that only later evolved the ability to synthesize proteins when amino acids began to appear.[47] Studies suggest that ancient ribosomes constructed solely of rRNA could have developed the ability to synthesize peptide bonds.[48][49][50] In addition, evidence strongly points to ancient ribosomes as self-replicating complexes, where the rRNA in the ribosomes had informational, structural, and catalytic purposes because it could have coded for tRNAs and proteins needed for ribosomal self-replication.[51] Hypothetical cellular organisms with self-replicating RNA but without DNA are called ribocytes (or ribocells).[52][53]
As amino acids gradually appeared in the RNA world under prebiotic conditions,[54][55] their interactions with catalytic RNA would increase both the range and efficiency of function of catalytic RNA molecules.[47] Thus, the driving force for the evolution of the ribosome from an ancient self-replicating machine into its current form as a translational machine may have been the selective pressure to incorporate proteins into the ribosome’s self-replicating mechanisms, so as to increase its capacity for self-replication.[51]
Specialized ribosomes[edit]
Heterogeneity in ribosome composition has been proposed to be involved in translational control of protein synthesis.[56] Vincent Mauro and Gerald Edelman proposed the ribosome filter hypothesis to explain the regulatory functions of ribosomes. Emerging evidence has shown that specialized ribosomes specific to different cell populations can affect how genes are translated.[57] Some ribosomal proteins exchange from the assembled complex with cytosolic copies [58] suggesting that the structure of the in vivo ribosome can be modified without synthesizing an entire new ribosome.
See also[edit]
References[edit]
- ^ Jones, Daniel (2003) [1917], Peter Roach, James Hartmann and Jane Setter, eds., English Pronouncing Dictionary, Cambridge: Cambridge University Press, ISBN 3-12-539683-2
- ^ Salini Konikkat: Dynamic Remodeling Events Drive the Removal of the ITS2 Spacer Sequence During Assembly of 60S Ribosomal Subunits in S. cerevisiae. Carnegie Mellon University Dissertations, February 2016.
- ^ Elmar W. Weiler, Lutz Nover (2008) (in German), [[1], p. 532, at Google Books Allgemeine und molekulare Botanik], Stuttgart: Georg Thieme Verlag, p. 532, ISBN 978-3-13-152791-2
- ^ Jesus de la Cruz, Katrin Karbstein, John L. Woolford, Jr. (2015), "Functions of Ribosomal Proteins in Assembly of Eukaryotic Ribosomes In Vivo" (in German), Annual review of biochemistry84: pp. 93–129, doi:10.1146/annurev-biochem-060614-033917, PMC 4772166, PMID 25706898
- ^ Benne R, Sloof P (1987). "Evolution of the mitochondrial protein synthetic machinery". BioSystems. 21 (1): 51–68. doi:10.1016/0303-2647(87)90006-2. PMID 2446672.
- ^ "Ribosomes". Retrieved 2011-04-28.
- ^ Palade, G.E. (January 1955). "A small particulate component of the cytoplasm". J Biophys Biochem Cytol. 1 (1): 59–68. doi:10.1083/jcb.1.1.59. PMC 2223592
 . PMID 14381428.
. PMID 14381428. - ^ Roberts, R. B., editor. (1958) "Introduction" in Microsomal Particles and Protein Synthesis. New York: Pergamon Press, Inc.
- ^ "The Nobel Prize in Physiology or Medicine 1974". Nobelprize.org. The Nobel Foundation. Retrieved 10 December2012.
- ^ "2009 Nobel Prize in Chemistry". The Nobel Foundation. Retrieved 10 December 2012.
- ^ Kurland, C.G. (1960). "Molecular characterization of ribonucleic acid from Escherichia coli ribosomes". Journal of Molecular Biology. 2 (2): 83–91. doi:10.1016/s0022-2836(60)80029-0.
- ^ Wilson, Daniel N.; Doudna Cate, Jamie H. (May 2012). "The Structure and Function of the Eukaryotic Ribosome". Cold Spring Harbor Perspectives in Biology. 4 (5): a011536. doi:10.1101/cshperspect.a011536. ISSN 1943-0264. PMC 3331703
 . PMID 22550233.
. PMID 22550233. - ^ Nissen P, Hansen J, Ban N, Moore PB, Steitz TA. The structural basis of ribosome activity in peptide bond synthesis. Science. 2000 Aug 11;289(5481):920-30. PMID 10937990
- ^ a b Wimberly BT, Brodersen DE, Clemons WM Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V (September 2000). "Structure of the 30S ribosomal subunit". Nature. 407 (6802): 327–39. doi:10.1038/35030006. PMID 11014182.
- ^ a b c d e f g The Molecular Biology of the Cell, fourth edition. Bruce Alberts, et al. Garland Science (2002) pg. 342 ISBN 0-8153-3218-1
- ^ Reginald Garrett and Charles M. Grisham: Biochemistry. (International Student Edition). Cengage Learning Services; 4th edition 2009; ISBN 978-0-495-11464-2; p. 962.
- ^ Czernilofsky, A; Küchler, E; Stöffler, G.; Czernilofsky, P. (1976). "SITE OF REACTION ON RIBOSOMAL-PROTEIN L27 WITH AN AFFINITY LABEL DERIVATIVE OF TRANSFER-RNA-F(MET)". FEBS Letters. ELSEVIER SCIENCE BV. 63 (2): 283–286. doi:10.1016/0014-5793(76)80112-3. PMID 770196.
- ^ Czernilofsky, A; Collatz, E; Stöffler, G; Küchler, E (1974). "PROTEINS AT TRANSFER-RNA BINDING-SITES OF ESCHERICHIA-COLI RIBOSOMES". Proceedings of the National Academy of Sciences of the United States of America. NATL ACAD SCIENCES. 71 (1): 230–234. doi:10.1073/pnas.71.1.230. PMC 387971
 . PMID 4589893.
. PMID 4589893. - ^ Czernilofsky, A; Kurland, C.G.; Stöffler, G. (1975). "30S RIBOSOMAL-PROTEINS ASSOCIATED WITH 3'-TERMINUS OF 16S RNA". FEBS Letters. ELSEVIER SCIENCE BV. 58 (1): 281–284. doi:10.1016/0014-5793(75)80279-1. PMID 1225593.
- ^ a b c Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M (February 2011). "The structure of the eukaryotic ribosome at 3.0 Å resolution". Science. 334 (6062): 1524–1529. doi:10.1126/science.1212642. PMID 22096102.
- ^ a b Rabl, Leibundgut, Ataide, Haag, Ban (February 2010). "Crystal Structure of the Eukaryotic 40S Ribosomal Subunit in Complex with Initiation Factor 1". Science. 331 (6018): 730–736. doi:10.1126/science.1198308. PMID 21205638.
- ^ a b Klinge, Voigts-Hoffmann, Leibundgut, Arpagaus, Ban (November 2011). "Crystal Structure of the Eukaryotic 60S Ribosomal Subunit in Complex with Initiation Factor 6". Science. 334 (6058): 941–948. doi:10.1126/science.1211204. PMID 22052974.
- ^ Reginald Garrett and Charles M. Grisham: Biochemistry. (International Student Edition). Cengage Learning Services; 4th edition 2009; ISBN 978-0-495-11464-2; p. 965.
- ^ Czernilofsky, A; Collatz, Ekkehard; Gressner, Axel M.; Wool, Ira G.; Küchler, Ernst (1977). "IDENTIFICATION OF TRNA-BINDING SITES ON RAT-LIVER RIBOSOMES BY AFFINITY LABELING". Molecular and General Genetics. Springer Verlag. 153 (3): 231–235. doi:10.1007/BF00431588. Retrieved 1 Jan2012.
- ^ Recht MI, Douthwaite S, Puglisi JD (1999). "Basis for bacterial specificity of action of aminoglycoside antibiotics". EMBO J. 18(11): 3133–8. doi:10.1093/emboj/18.11.3133. PMC 1171394
 . PMID 10357824.
. PMID 10357824. - ^ O'Brien, T.W. (1971). "The General Occurrence of 55S Ribosomes in Mammalian Liver Mitochondria". J. Biol. Chem. 245: 3409.
- ^ a b Ban N, Nissen P, Hansen J, Moore P, Steitz T (2000). "Å". Science. 289 (5481): 905–20. doi:10.1126/science.289.5481.905. PMID 10937989.
- ^ Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehmann M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A (September 1, 2000). "Å". Cell. 102 (5): 615–23. doi:10.1016/S0092-8674(00)00084-2. PMID 11007480.
- ^ Yusupov MM, Yusupova GZ, Baucom A, et al. (May 2001). "Crystal structure of the ribosome at 5.5 A resolution". Science. 292 (5518): 883–96. doi:10.1126/science.1060089. PMID 11283358.
- ^ Schuwirth BS, Borovinskaya MA, Hau CW, et al. (November 2005). "Structures of the bacterial ribosome at 3.5 A resolution". Science. 310 (5749): 827–34. doi:10.1126/science.1117230. PMID 16272117.
- ^ Mitra K, Schaffitzel C, Shaikh T, et al. (November 2005). "Structure of the E. coli protein-conducting channel bound to a translating ribosome". Nature. 438 (7066): 318–24. doi:10.1038/nature04133. PMC 1351281
 . PMID 16292303.
. PMID 16292303. - ^ Selmer M, Dunham CM, Murphy FV, et al. (September 2006). "Structure of the 70S ribosome complexed with mRNA and tRNA". Science. 313 (5795): 1935–42. doi:10.1126/science.1131127. PMID 16959973.
- ^ Korostelev A, Trakhanov S, Laurberg M, Noller HF (September 2006). "Crystal structure of a 70S ribosome-tRNA complex reveals functional interactions and rearrangements". Cell. 126 (6): 1065–77. doi:10.1016/j.cell.2006.08.032. PMID 16962654.
- ^ Yusupova G, Jenner L, Rees B, Moras D, Yusupov M (November 2006). "Structural basis for messenger RNA movement on the ribosome". Nature. 444 (7117): 391–4. doi:10.1038/nature05281. PMID 17051149.
- ^ Savir, Y; Tlusty, T (Apr 11, 2013). "The ribosome as an optimal decoder: a lesson in molecular recognition". Cell. 153 (2): 471–9. doi:10.1016/j.cell.2013.03.032. PMID 23582332.
- ^ Konevega, AL; Soboleva, NG; Makhno, VI; Semenkov, YP; Wintermeyer, W; Rodnina, MV; Katunin, VI (Jan 2004). "Purine bases at position 37 of tRNA stabilize codon-anticodon interaction in the ribosomal A site by stacking and Mg2+-dependent interactions". RNA. 10 (1): 90–101. doi:10.1261/rna.5142404. PMC 1370521
 . PMID 14681588.
. PMID 14681588. - ^ Rodnina, M.V.; Beringer, M.; Wintermeyer, W. (January 2007). "How ribosomes make peptide bonds". Trends Biochem. Sci. 32(1): 20–6. doi:10.1016/j.tibs.2006.11.007. PMID 17157507.
- ^ Cech, T. (August 11, 2000). "Structural biology. The Ribosome Is a Ribozyme". Science. 289 (5481): 878–9. doi:10.1126/science.289.5481.878. PMID 10960319.
- ^ Brandman O, et al. (2012). "A ribosome-bound quality control complex triggers degradation of nascent peptides and signals translation stress". Cell. 151 (5): 1042–54. doi:10.1016/j.cell.2012.10.044. PMC 3534965
 . PMID 23178123.
. PMID 23178123. - ^ Defenouillère Q, et al. (2013). "Cdc48-associated complex bound to 60S particles is required for the clearance of aberrant translation products". Proc Natl Acad Sci U S A. 110 (13): 5046–51. doi:10.1073/pnas.1221724110. PMC 3612664
 . PMID 23479637.
. PMID 23479637. - ^ Shen PS, et al. (2015). "Rqc2p and 60S ribosomal subunits mediate mRNA-independent elongation of nascent chains". Science. 347 (6217): 75–8. doi:10.1126/science.1259724. PMC 4451101
 . PMID 25554787.
. PMID 25554787. - ^ Keeley, Jim; Gutnikoff, Robert (2015-01-02). "Ribosome Studies Turn Up New Mechanism of Protein Synthesis" (Press release). Howard Hughes Medical Institute. Retrieved 2015-01-16.
- ^ University of Würzburg. (2016, October 6). Ribosomal quality control. ScienceDaily. https://www.sciencedaily.com/releases/2016/10/161006122726.htm
- ^ http://newsrescue.com/ribosomal-quality-control/#axzz4qxPAgyHU
- ^ https://phys.org/news/2016-10-ribosomal-quality.html
- ^ https://www.ncbi.nlm.nih.gov/books/NBK26841/#A2204
- ^ a b Noller, H. F. (2012). "Evolution of protein synthesis from an RNA world". Cold Spring Harbor Perspectives in Biology. 4: 1–U20. doi:10.1101/cshperspect.a003681. PMC 3312679
 . PMID 20610545.
. PMID 20610545. - ^ Dabbs, E.R. (1986). Mutant studies on the prokaryotic ribosome. Springer-Verlag, N.Y.
- ^ Noller, H. F.; Hoffarth, V.; Zimniak, L. (June 5, 1992). "Unusual resistance of peptidyl transferase to protein extraction procedures". Science. 256: 1416–1419. doi:10.1126/science.1604315. PMID 1604315.
- ^ Nomura, M.; Mizushima, S.; Ozaki, M.; Trau, P.; Lowry, C. V. (1969). "Structure and function of ribosomes and their molecular components". Cold Spring Harbor Symposium of Quantitative Biology. 34: 49–61. doi:10.1101/sqb.1969.034.01.009.
- ^ a b Root-Bernstein, M.; Root-Bernstein, R. (2015). "The ribosome as a missing link in the evolution of life". Journal of Theoretical Biology. 367: 130–158. doi:10.1016/j.jtbi.2014.11.025. PMID 25500179.
- ^ Yarus M (2002). "Primordial genetics: phenotype of the ribocyte". Annu. Rev. Genet. 36: 125–51. doi:10.1146/annurev.genet.36.031902.105056. PMID 12429689.
- ^ P. Forterre, M. Krupovic: The Origin of Virions and Virocells: The Escape Hypothesis Revisited. In: G. Witzany (Ed.): Viruses: Essential Agents of Life, Seite 43-60, Springer Link, September 25, 2012. ISBN 978-94-007-4898-9 (Print) 978-94-007-4899-6 (Online), DOI 10.1007/978-94-007-4899-6
- ^ Caetano-Anolles, G.; Seufferheld, M. J. (2013). "The coevolutionary roots of biochemistry and cellular organization challenge the RNA world paradigm". Journal of Molecular Microbiology and Biotechnology. 23: 152–177. doi:10.1159/000346551.
- ^ Saladino, R.; Botta, G.; Pino, S.; Costanzo, G.; Mauro, E. Di (2012). "Genetics first or metabolism first? The formamide clue". Chemical Society Reviews. 41: 5526–5565. doi:10.1039/c2cs35066a.
- ^ Mauro, Vincent P.; Edelman, Gerald M. (2002-09-17). "The ribosome filter hypothesis". Proceedings of the National Academy of Sciences of the United States of America. 99 (19): 12031–12036. doi:10.1073/pnas.192442499. ISSN 0027-8424. PMC 129393
 . PMID 12221294.
. PMID 12221294. - ^ Xue, Shifeng; Barna, Maria (May 23, 2012). "Specialized ribosomes: a new frontier in gene regulation and organismal biology". Nature Reviews Molecular Cell Biology. 13 (6): 355–369. doi:10.1038/nrm3359. ISSN 1471-0080. PMC 4039366
 . PMID 22617470.
. PMID 22617470. - ^ Mathis, Andrew (8 Dec 2016). "Mechanisms of in vivo ribosome maintenance change in response to nutrient signals". Molecular & Cellular Proteomics. mcp.M116.063255.: 243–254. doi:10.1074/mcp.M116.063255. PMC 5294211
 . PMID 27932527.
. PMID 27932527.





No comments:
Post a Comment