Polyphenol
From Wikipedia, the free encyclopedia
| This article may be too technical for most readers to understand. (July 2016) (Learn how and when to remove this template message) |
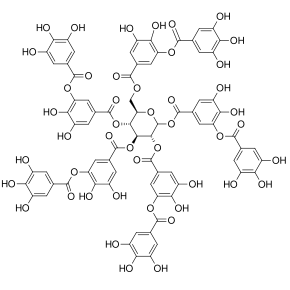
Polyphenols[1][2] (noun, pronunciation of the singular /pɒliˈfiːnəl/[3] or /pɒliˈfɛnəl/; also known as polyhydroxyphenols) are a structural class of mainly natural, but also synthetic or semisynthetic, organic chemicals characterized by the presence of large multiples of phenol structural units. The number and characteristics of these phenol structures underlie the unique physical, chemical, and biological (metabolic, toxic, therapeutic, etc.) properties of particular members of the class. Examples include tannic acid (image at right) and ellagitannin (image below). The historically important chemical class of tannins is a subset of the polyphenols.[1][4]
The name derives from the Ancient Greek word πολύς (polus, meaning "many, much") and the word phenol which refers to a chemical structure formed by attaching to an aromatic benzenoid (phenyl) ring, an hydroxyl (-OH) group akin to that found in alcohols (hence the -ol suffix). The term polyphenol appears to have been in use since 1894.[3]
Definition of the term polyphenol[edit]
Original "WBSSH" definition of polyphenols[edit]
The earliest widely accepted definition of polyphenols, the White–Bate-Smith–Swain–Haslam (WBSSH) definition,[5] was offered and justified by natural product and organic chemist Edwin Haslam and co-workers, based on the earlier natural products research of Edgar Charles Bate-Smith, Anthony Swain, and Theodore White that characterized specific structural characteristics common to plant phenolics used in tanning (i.e., the tannins).[6] The WBSSH describes the polyphenol class as:
- generally moderately water-soluble compounds
- with molecular weight of 500–4000 Da
- with >12 phenolic hydroxyl groups
- with 5–7 aromatic rings per 1000 Da
where the limits to these ranges are somewhat flexible.[1][5] The definition further states that polyphenols display unique physical and chemical behaviors related to their high molecular weights and profusion of phenolic substructures—precipitation of proteins and particular amine-containing organics (e.g., particular alkaloid natural products), and formation of particular metal complexes (e.g., intense blue-black iron(III) complexes).
Proposed Quideau definition of polyphenols[edit]
The need to clarify the definition of 'polyphenols' in the light of the extensive research into this large substance class and of increasingly ambiguous use of the polyphenol term led Stéphane Quideau, Bordeaux 1 University, France, to offer a definition not given formal status by IUPAC:[2]
- The term "polyphenol" should be used to define compounds exclusively derived from the shikimate/phenylpropanoid and/or the polyketide pathway, featuring more than one phenolic unit and deprived of nitrogen-based functions.
Structurally, this definition continues to steer the definition away from exclusively man-made structures without corresponding natural products, and explicitly excludes monophenolic structures (man-made or naturally occurring) and their derivatives, e.g., phenyl esters, methyl phenyl ethers and O-phenyl glycosides. This definition departs from the WBSSH definition in terms of physicochemical behavior, with its lack of reference to solubility, precipitation, and complexation phenomena.
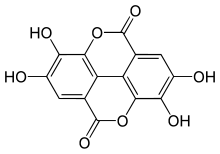
The gallic acid dimer, ellagic acid (M.W. 302, right), a molecule at the core of naturally occurring phenolic compounds of varying sizes, is itself not a polyphenol by the WBSSH definition, but is by the Quideau definition. The raspberry ellagitannin (M.W. ~2450),[7] on the other hand, with its 14 gallic acid moieties (most in ellagic acid-type components), and more than 40 phenolic hydroxyl groups, meets the criteria of both definitions of a polyphenol. Other examples of compounds that fall under both the WBSSH and Quideau definitions include the black tea antioxidant theaflavin-3-gallate shown below, and the hydrolyzable tannin, tannic acid, shown above.
Defining chemical reactions of the polyphenol class[edit]
Individual polyphenols engage in reactions related to both their core phenolic structures, their linkages, and types of glycosidesthey form. Standard phenolic reactions include ionization (which contributes to solubility and complexation), oxidations to ortho- and para-quinones (which contributes to antioxidant characteristics), and underlying aromatic transformations related to the presence of the phenolic hydroxyl (see phenol image above); reactions related to their linkages include nucleophilic additions, and oxidative and hydrolytic bond cleavages.[8] In addition, as noted above, a traditional feature of polyphenols was their ability to form particular, characteristic metal complexes.[5]
Chemical structure and synthesis[edit]
Structural features[edit]
As opposed to smaller phenols, polyphenols are often larger molecules (macromolecules) deposited in cell vacuoles. The upper molecular weight limit for small molecules is about 800 Daltons, which allows for the possibility to rapidly diffuse across cell membranes so that they can reach intracellular sites of action or remain as pigments once the cell senesces. Hence, many larger polyphenols are biosynthesized in-situ from smaller polyphenols to nonhydrolyzable tannins and remain undiscovered in the plant matrix. Most polyphenols contain repeating phenolic moieties of pyrocatechol, resorcinol, pyrogallol, and phloroglucinol connected by esters (hydrolyzable tannins) or more stable C-C bonds (nonhydrolyzable condensed tannins). Proanthocyanidins are mostly polymeric units of catechin and epicatechin. Catechol and resorcinol (benzenediol) types of polyphenols have two, and pyrogallol and phloroglucinol (benzenetriol) types have three phenolic hydroxyl groups, respectively, though mixing of these types within polyphenols is also possible. The phenolic substructures arise from various biosynthetic pathways (WBSSH definition), especially phenylpropanoid and polyketide branches aimed at plant and related secondary metabolites (both definitions).
 |  |  |  |
Polyphenols always have heteroatom substituents other than hydroxyl groups; ether and ester linkages are common, as are various carboxylic acid derivatives (see theaflavin gallate image); ester linkages are common in the hydrolyzable tannins. Apart from simple heteroatom links, the carbon frameworks can become complex, e.g., various carbon-carbon bond linkages join hydrolytically labile esters and ethers as common in non-hydrolyzable condensed tannins.
In these, diverse biosynthetic steps abound: the seven-atom ring (seven-membered ring) appearing in theaflavin structure above is an example of a "carbocycle" that is of a nonbenzenoid aromatic tropolone type. In addition, there are periodic occurrences of:
- benzopyrans and normal and C-glucoside derivatives (figure at right)—e.g. in condensed, complex and hydrolyzable tannins such as in stenophyllanin A, acutissimin B, mongolicain A, stenophynin A, mongolicanin, and mongolicin B,
- various biaryls and triaryls (e.g., biphenyls), see further figure at right,
- spiro-type structures as illustrated at right, e.g., in mongolicain A,
- furanoid, pyrone, and other heterocycles,
- (diaryl)methyl structures,
- pyrans and dioxins, etc.[4]
Because of the preponderance of saccharide-derived core structures (e.g., see tannic acid image above), as well as spiro- and other structure types, natural chiral (stereo) centers abound.
Chemical synthesis[edit]
True polyphenols from the tannin and other WBSSH types are routinely biosynthesized in the natural sources from which they derive; their 'chemical' syntheses (using standard "bench" organic chemical methods) were somewhat limited until the first decade of the new millennium because these syntheses involve challenging regioselectivity and stereoselectivity issues.[9]Early work focused on the achiral synthesis of phenolic-related components of polyphenols in the late 70's,[10] and the Nelson and Meyers synthesis of the permethyled derivative of the ubiquitous diphenic acid core of ellagitannins in 1994[11] followed by stereoselective synthesis of more complex permethylated structures such as a (+)-tellimagrandin II derivative by Lipshutz and coworkers in the same year,[12] and Itoh and coworker's synthesis of a permethylated pedunculagin with particular attention to axial symmetry issues in 1996.[13] The total synthesis of a fully unmasked polyphenol, that of the ellagitannin tellimagrandin I, was a diastereoselective sequence reported in 1994 by Feldman, Ensel and Minard.[14]
Further total syntheses of deprotected polyphenols that followed were led by the Feldman group, for instance in Feldman and Lawlor's synthesis of the ellagitannin, coriariin A and other tannin relatives.[15] Khanbabaee and Grosser accomplished a relatively efficient total synthesis of pedunculagin in 2003.[16][17]
Work proceeded with focus on enantioselective total syntheses, e.g., on atroposelective syntheses of axially chiral biaryl polyphenols,[18][19] with recent further important work including controlled assembly of a variety of polyphenols according to integrated strategies, such as in syntheses of extended series of procyanidins (oligomeric catechins) by various groups[20] and of resveratrol polyphenols by the Snyder group at Columbia that included the diverse carasiphenols B and C, ampelopsins G and H, and nepalensinol B.[21][22] A biomimetic synthesis, and the first formal total synthesis 5-O-Desgalloyl-epi-punicacortein A, a further ellagitannin in its C-glucosyl (C-glucoside subclass), has also recently been accomplished.[23] The novel strategies and methods referred to in these recent examples helped to open the field of polyphenol chemical synthesis to an unprecedented degree.[22]
Chemical properties and uses[edit]
Chemical properties[edit]
Polyphenols are molecules owing their UV/Vis absorptivity to aromatic structures with large conjugated systems of pi electronconfigurations; they also have autofluorescence properties, especially lignin and the phenolic part of suberin.[24]
They are reactive species toward oxidation.[25] ABTS may be used to characterise polyphenol oxidation products.[26]
Polyphenols also characteristically possess a significant binding affinity for proteins, which can lead to the formation of soluble and insoluble protein-polyphenol complexes.[27]
Chemical uses[edit]
Some polyphenols are traditionally used as dyes. For instance, in the Indian subcontinent, the pomegranate peel, high in tannins and other polyphenols, or its juice, is employed in the dyeing of non-synthetic fabrics.[28]
Polyphenols, especially tannins, were used traditionally for tanning leather and today also as precursors in green chemistry[29]notably to produce plastics or resins by polymerisation with[30] or without the use of formaldehyde[31] or adhesives for particleboards.[32] The aims are generally to make use of plant residues from grape, olive (called pomaces) or pecan shells left after processing.[33]
Cashew nut shell liquid (CNSL) is an important phenolic raw material containing mostly cardol, cardanol and anacardic acid. Strictly speaking not a polyphenol, it is used mainly in polymer-based industries for friction linings, paints, varnishes, laminating resins, rubber compounding resins, polyurethane based polymers, surfactants, epoxy resins and wood preservatives.[34]
Pyrogallol and pyrocatechin are among the oldest photographic developers.[35]:25
Biology[edit]
Biological role in plants[edit]
Both natural phenols and the larger polyphenols play important roles in the ecology of most plants. Their effects in plant tissues can be divided into the following categories:[36]
- Release and suppression of growth hormones such as auxin.
- UV screens to protect against ionizing radiation and to provide coloration (plant pigments).
- Deterrence of herbivores (sensory properties).
- Prevention of microbial infections (phytoalexins).[37]
- Signaling molecules in ripening and other growth processes.
Occurrence in nature[edit]
The most abundant polyphenols are the condensed tannins, found in virtually all families of plants. Larger polyphenols are often concentrated in leaf tissue, the epidermis, bark layers, flowers and fruits but also play important roles in the decomposition of forest litter, and nutrient cycles in forest ecology. Absolute concentrations of total phenols in plant tissues differ widely depending on the literature source, type of polyphenols and assay; they are in the range of 1-25% total natural phenols and polyphenols, calculated with reference to the dry green leaf mass.[38]
High levels of polyphenols in some woods can explain their natural preservation against rot.[39]
Flax and Myriophyllum spicatum (a submerged aquatic plant) secrete polyphenols that are involved in allelopathic interactions.[40][41]
Polyphenols are also found in animals. In arthropods such as insects[42] and crustaceans[43] polyphenols play a role in epicuticle hardening (sclerotization). The hardening of the cuticle is due to the presence of a polyphenol oxidase.[44] In crustaceans, there is a second oxidase activity leading to cuticle pigmentation.[45] There is apparently no polyphenol tanning occurring in arachnids cuticle.[46]
Metabolism[edit]
Biosynthesis and metabolism[edit]
Polyphenols incorporate smaller parts and building blocks from simpler natural phenols, which originate from the phenyl propanoid pathway for the phenolic acids or the shikimic acid pathway for gallotannins and analogs. Flavonoids and caffeic acid derivatives are biosynthesized from phenyl alanine and malonyl-CoA. Complex gallotannins develop through the in-vitro oxidation of 1,2,3,4,6-pentagalloyl-glucose or dimerization processes resulting in hydrolyzable tannins. For anthocyanidins, precursors of the condensed tannin biosynthesis, dihydroflavonol reductase and leucoanthocyanidin reductase (LAR) are crucial enzymes with subsequent addition of catechin and epicatechin moieties for larger, non-hydrolyzable tannins.[47]
The glycosylated form develops from glucosyltransferase activity and increases the solubility of polyphenols.[48]
Polyphenol oxidase (PPO) is an enzyme that catalyses the oxidation of o-diphenols to produce o-quinones. It is the rapid polymerisation of o-quinones to produce black, brown or red polyphenolic pigments that is the cause of fruit browning. In insects, PPO serves for the cuticle hardening.[49]
Laccase is a major enzyme that initiates the cleavage of hydrocarbon rings, which catalyzes the addition of a hydroxyl group to phenolic compounds. This enzyme can be found in fungi like Panellus stipticus, organisms able to break down lignin, a complex aromatic polymer in wood that is highly resistant to degradation by conventional enzyme systems.
Content in food[edit]
Generally foods contain complex mixtures of polyphenols.[52] According to a 2005 review on polyphenols:
Some polyphenols are considered antinutrients, compounds that interfere with the absorption of essential nutrients, especially iron and other metal ions, but also by binding to digestive enzymes and other proteins, particularly in ruminants.[53]
Phenolic and carotenoid compounds with antioxidant properties in vegetables have been found to be retained significantly better through steaming than through frying.[54]
Polyphenols in wine, beer and various nonalcoholic juice beverages can be removed using finings, substances that are usually added at or near the completion of the processing of brewing.
Potential health effects[edit]
Many polyphenolic extracts, for example from grape skin, grape seeds, olive pulp and maritime pine bark are sold as ingredients in functional foods, dietary supplements and cosmetics without any legal health claims. Some of them have self-affirmed GRAS status in the US. There are no recommended Dietary Reference Intake levels established for polyphenols.[55]
The diverse structures of phenolic compounds prohibit broad statements about their specific health effects. Further, many purported health claims for specific polyphenol-enriched foods remain unproven.[56] Many of the phytoestrogens are dietary polyphenols with measurable affinities to estrogen receptors, and positive or negative health effects on humans and livestock.[57]
Compared with the effects of polyphenols in vitro, the effects in vivo, although the subject of ongoing research, are limited and vague. The reasons for this are 1) the absence of validated in vivo biomarkers, especially for inflammation or carcinogenesis; 2) long-term studies failing to demonstrate effects with a mechanism of action, specificity or efficacy; and 3) invalid applications of high, unphysiological test concentrations in the in vitro studies, which are subsequently irrelevant for the design of in vivo experiments.[58]
A review of studies on the bioavailability of polyphenols published in 2010 found that "definitive conclusions on bioavailability of most polyphenols are difficult to obtain and further studies are necessary."[52]
Traditional medicine[edit]
Many herbal teas contain soluble polyphenols, and their efficacy is often attributed to astringent substances.[59] In the Ayurveda system of medicine for example, the pomegranate has extensively been used as a source of traditional remedies.[28]
Research techniques[edit]
Sensory properties[edit]
With respect to food and beverages, the cause of astringency is not fully understood, but it is measured chemically as the ability of a substance to precipitate proteins.[60]
A review published in 2005 found that astringency increases and bitterness decreases with the mean degree of polymerization. For water-soluble polyphenols, molecular weights between 500 and 3000 were reported to be required for protein precipitation. However, smaller molecules might still have astringent qualities likely due to the formation of unprecipitated complexes with proteins or cross-linking of proteins with simple phenols that have 1,2-dihydroxy or 1,2,3-trihydroxy groups.[61]Flavonoid configurations can also cause significant differences in sensory properties, e.g. epicatechin is more bitter and astringent than its chiral isomer catechin. In contrast, hydroxycinnamic acids do not have astringent qualities, but are bitter.[62]
Analysis[edit]
The analysis techniques are those of phytochemistry: extraction, isolation, structural elucidation,[63] then quantification.
Extraction[edit]
Extraction of polyphenols[64] can be performed using a solvent like water, hot water, methanol, methanol/formic acid, methanol/water/acetic or formic acid etc. Liquid liquid extraction can be also performed or countercurrent chromatography. Solid phase extraction can also be made on C18 sorbent cartridges. Other techniques are ultrasonic extraction, heat reflux extraction, microwave-assisted extraction,[65] critical carbon dioxide,[33][66] pressurized liquid extraction[67] or use of ethanol in an immersion extractor.[68] The extraction conditions (temperature, extraction time, ratio of solvent to raw material, solvent and concentrations) have to be optimized.
Mainly found in the fruit skins and seeds, high levels of polyphenols may reflect only the measured extractable polyphenol (EPP) content of a fruit which may also contain non-extractable polyphenols. Black tea contains high amounts of polyphenol and makes up for 20% of its weight.[69]
Concentration can be made by ultrafiltration.[70] Purification can be achieved by preparative chromatography.
Analysis techniques[edit]
Phosphomolybdic acid is used as a reagent for staining phenolics in thin layer chromatography. Polyphenols can be studied by spectroscopy, especially in the ultraviolet domain, by fractionation or paper chromatography. They can also be analysed by chemical characterisation.
Instrumental chemistry analyses include separation by high performance liquid chromatography (HPLC), and especially by reversed-phase liquid chromatography (RPLC), can be coupled to mass spectrometry.[33] Purified compounds can be identified by the means of nuclear magnetic resonance.
Microscopy analysis[edit]
The DMACA reagent is an histological dye specific to polyphenols used in microscopy analyses. The autofluorescence of polyphenols can also be used, especially for localisation of lignin and suberin.
Quantification[edit]
Polyphenolic content can be quantified separation/isolation by volumetric titration. An oxidizing agent, permanganate, is used to oxidize known concentrations of a standard tannin solution, producing a standard curve. The tannin content of the unknown is then expressed as equivalents of the appropriate hydrolyzable or condensed tannin.[71]
Some methods for quantification of total polyphenol content are based on colorimetric measurements. Some tests are relatively specific to polyphenols (for instance the Porter's assay). Total phenols (or antioxidant effect) can be measured using the Folin-Ciocalteu reaction.[33] Results are typically expressed as gallic acid equivalents. Polyphenols are seldom evaluated by antibody technologies.[72]
Other tests measure the antioxidant capacity of a fraction. Some make use of the ABTS radical cation which is reactive towards most antioxidants including phenolics, thiols and vitamin C.[73] During this reaction, the blue ABTS radical cation is converted back to its colorless neutral form. The reaction may be monitored spectrophotometrically. This assay is often referred to as the Trolox equivalent antioxidant capacity (TEAC) assay. The reactivity of the various antioxidants tested are compared to that of Trolox, which is a vitamin E analog.
Other antioxidant capacity assays which use Trolox as a standard include the diphenylpicrylhydrazyl (DPPH), oxygen radical absorbance capacity (ORAC),[74] ferric reducing ability of plasma (FRAP)[75] assays or inhibition of copper-catalyzed in vitro human low-density lipoprotein oxidation.[76]
New methods including the use of biosensors can help monitor the content of polyphenols in food.[77]
Quantitation results produced by the mean of diode array detector-coupled HPLC are generally given as relative rather than absolute values as there is a lack of commercially available standards for all polyphenolic molecules.
See also[edit]
- Polyphenolic proteins
- List of antioxidants in food
- List of phytochemicals in food
- Nutrition
- Phytochemistry
- Secondary metabolites
- Oligostilbenoids
- Polyphenol: A kind of chemical that (at least in theory) may protect against some common health problems and possibly certain effects of aging.Polyphenols act as antioxidants. They protect cells and body chemicals against damage caused by free radicals, reactive atoms that contribute to tissue damage in the body. For example, when low-density lipoprotein (LDL) cholesterol is oxidized, it can become glued to arteries and cause coronary heart disease.Polyphenols can also block the action of enzymes that cancers need for growth and they can deactivate substances that promote the growth of cancers. The polyphenol most strongly associated with cancer prevention is epigallocatechin-3-gallate, or EGCG.All tea contains polyphenols. Teas and polyphenols isolated from tea have been shown in the laboratory to act as scavengers of oxygen and nitrogen-free radicals, protecting the fatty membranes of cells, proteins and DNA. However, the results of human studies of tea and polyphenols to date (2001) have been inconsistent and have yet to prove anything one way or the other as regards the value of polyphenols.
10 Best Polyphenol-Rich Superfoods + Why You Should Be Eating Them
"Eat food, not too much, mostly plants." The simplicity and accuracy of those words, written by Michael Pollan in his masterpiece In Defense of Food, are unmatched.
But why are plant-based foods so powerful? Why do I repeat over and over to my heart patients to get seven to 10 servings a day of brightly colored fruits and vegetables? For example, just this week the news channels reported a story that blueberries improve both blood pressure and the healthy flexibility of arteries, making them act younger.
The secret that blueberries and other selected whole foods possess is that they are rich sources of polyphenols. Let's get to know these powerful chemicals a bit more.
Time to pay attention for a one-minute food chemistry lesson. Polyphenols are a group of plant-based chemicals that have at least one phenol group (don't ask me why we don't call some monophenols — I don't know). One broad type of polyphenols are phenolic acids including red fruits, black radishes, onions, coffees, cereals and spices.
The second broad group are the flavonoids, including isoflavones found in soy, anthocyanidins found in berries and wine, flavones found in herbs, flavonols found in broccoli, tomato and tea, flavanones found in citrus fruits and juices, and flavan-3-ols found in cocoa, tea and wine.
Finally, some famous ones don't fit into any class, including resveratrol and stilbenes from wine and nuts, curcumin in spices, and lignans in flaxseeds.
Polyphenols improve your health in six ways:
- Lower cholesterol
- Lower blood pressure
- Improve artery (endothelial) function
- Prevent platelet clumping
- Improve arterial flexibility
- Improved life span
The evidence for the heart benefits for foods rich in polyphenols comes from hundreds of studies. One example published last year was a large study in Europe reporting that a higher intake of polyphenols, particularly stilbenes from grapes and nuts and lignans from flax, was associated with a longer life span.
In another recent study, of more than 500 European subjects, healthier arteries were found in those who ate raw vegetables and avoided high-fat dairy products. Consumption of fresh fruit, wine and avoidance of high-fat dairy products was also associated with less inflammation in the same subjects.
Still need more? In over 34,000 post-menopausal women, intake of flavonoid-rich foods such as bran, apples, pears, grapefruit, strawberries, red wine and chocolate was associated with a lower risk of heart disease and all-cause deaths.
Want a few extra tips? The top 100 richest foods in polyphenols has been studied and a list was published, but the top 10 are:
- Cloves
- Star anise
- Cocoa powder
- Mexican oregano, dried
- Celery seed
- Black chokeberry
- Dark chocolate
- Flaxseed meal
- Black elderberry
- Chestnut
Honorable mention goes to sage, rosemary, spearmint, thyme, capers, basil, curry, strawberries and coffee.
There is no doubt that food is information. Food can act as a natural medicine, your dinner can determine your destiny, and your fork can decide your fate. Polyphenol-rich foods found in fruits, vegetables, nuts and seeds are a pharmacy to enhance your bodies "chemistry set", moving the needle away from inflammation and disease and toward healing and vitality. As the deputy director of the USDA said in a rare moment of candor "Eat your damn vegetables," a path to health, energy and a long life free of illness can be found at the end of your intelligently placed fork.










No comments:
Post a Comment