Gut Microbiota and Obesity
Contents
[hide]Introduction
Obesity is a pathological condition that is becoming prevalent throughout the world[3]. It is a popular subject of research as obesity is found to be associated with cardiovascular diseases, type2 diabetes and cancer[4]. Recent studies have revealed a whole new insight into obesity, suggesting the link between intestinal microbes and weight gain[3]. Dietary variation and caloric intake induce changes in gut microbiome composition, selectively promoting the growth of certain microbes[5]. These gut microbes thrive and impact the host metabolism by affecting inflammation and fat storage[7,12]. Detailed knowledge of these intestinal signaling pathways is valuable to the development of possible treatments to obesity.
Human Gastrointestinal Tract
The human body is a large reservoir of microbial cells as microbes outnumber the amount of body cells by 10 times[1]. Gastrointestinal tract with pH of 5.7 to 6.7 and temperature of 37-37.2 ºC provides the optimal growth condition for many microorganism[15,16]. It houses over 100 trillion (1014) microbes, with 3.3 million unique microbial genes[1]. Over 99% of gut bacteria are anaerobes and this is due to limited oxygen supplies in the gut[1]. Most microbes are packed in the colon, with a density of 10^11-10^12 cells/ml2. Firmicutes and Bacteriodetes are the two dominating divisions in the gut, comprising more than 1000 bacterial species[1]. These gut bacteria will be the key players in regulating gut metabolism, and are critical in understanding metabolism dysfunctions.
Key Organisms
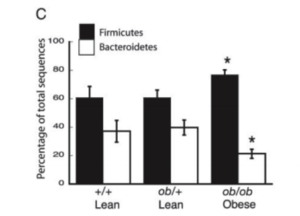
Most gut microbial species belong to four major phyla: Firmicutes, Actinobacteria, Proteobacteria and Bacteroidetes. Changes in the ratio of Firmicutes and Bacteroidetes correlates with obesity[5].
Genomic sequencings of bacterial rRNA from mice and human reveal the correlation between obesity and relative amount of these two bacterial phyla[6]. A high-fat diet promotes an increase of Firmicutes and relative reduction of Bacteroidete. The shift in dominating bacteria promotes more effective caloric intake that leads to the gaining of weight and obesity[5,6]. Likewise, when obese individual shift to a fat-restricted diet and body weight decreases, an increase of Bacteroidetes is observed[6]. This evidence suggests that an increase of Firmicutes and decrease of Bacteroidetes contribute to obesity and confirms the role of gut microbiota in regulating fat metabolism.
Effects on inflammation
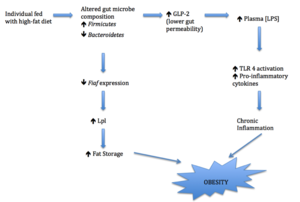
Shift in gut microbiome leads to chronic inflammation by increasing plasma LPS and results in obesity[5]. As mentioned previously, fatty acids in high-fat diet increases the amount of Firmicutes while reducing the amount of Bacteroidetes[5,6]. This alteration of the dominating bacterial phylum reduces the endogenous production of GLP-2. GLP-2 is an intestinal peptide that enhances tight junctions of the cell and prevents lipopolysaccharide (LPS) from entering plasma[11]. Thus, the decrease of GLP-2 as a result of altered gut microbe composition (shifting to Firmicutes due to high-fat diet) will lead to an increase in gut permeability and plasma LPS concentration [10]. In addition to the effect of GLP-2, LPS-containing Firmicutessignificantly contribute to LPS concentrations as well [9]. The increase of plasma LPS is significant as more LPS can activate TLR4 and upregulate the expression of pro-inflammatory cytokines [8]. As obesity is considered a chronic inflammatory disease, the increased production of pro-inflammatory cytokines will trigger an inflammatory response that ultimately leads to weight gain [7].
Effects on fatty acid metabolism and fat absorption
Gut microbes affect the regulation of energy metabolism by controlling the transcription of many intestinal mediators. It is found that when an individual is fasting, the expression of Fasting-induced adipose factor (Fiaf) in intestinal epithelial cells is elevated [3]. Fiaf (also known as angiopoietin-like protein 4), is produced by intestines, adipose tissues and human liver [12]. It inhibits the activity of lipoprotein lipase (Lpl), a regulatory enzyme that enhances the absorption of fatty acid and the build-up of adipocyte triglyceride [3]. Comparisons of germ-free and normal (colonization of gut microbiota) mice fed with high-fat diet show that the expression of epithelial Fiaf decreases by 9-fold with presence of gut bacteria [12]. This suggests that intestinal microbes altered by high-fat diet suppress Fiaf expression, promoting Lpl activity and increasing fat storage [12]. While gut microbes indeed alter the absorption of fat in the Fiaf-dependent pathway, direct evidence proving involvement of the two dominating bacteria phyla, Firmicutes and Bacteroidetes, in this signaling pathway is still elusive.
Current Research: Modulation of Gut microbiota in the management of metabolic diseases
The critical role of gut bacteria in metabolism is suggested by various mechanisms, such as induction of chronic inflammatory response in the GLP-2-dependent pathway and Fiaf-regulated fat absorption. Alteration or restoration of gut microbiota composition may possibly contribute to the pharmacotherapy of metabolic diseases and alleviate obesity.
Use of antibiotics
Antibiotics are commonly used when an imbalance of microflora or dismicrobism occur in the intestines to re-modulate the composition [11]. Antibiotics, such as Rifaximin, with low microbial resistance, non-absorbable property and few minor side effects such as headaches are ideal for the treatment of obesity [11].
Use of probiotics and prebiotics
Probiotics are viable microorganisms that can be delivered orally and incorporated into intestinal microbiome [14]. They are therapeutic agents that serve to benefit the health of the host [14]. Taken together with antibiotics, the adverse effects of both drugs can be alleviated and restorations of gut microflora can be enhanced [11].
Prebiotics are non-digestible food components that promote the growth of beneficial gut microbes [11]. Not only do prebiotics modulate gut microbes, they maintain appropriate levels of inflammation and intestinal peptide secretion as well [11]. Regular intake of prebiotics increases the fermentation of gut bacteria, reducing appetite and caloric intake and increasing glucose tolerance [13]. Today, prebiotics are valuable agents in pharmacotherapy of metabolic dysfunction [13].
References
[1] Zhu, B., Wang, X. and Li, L. “Human gut microbiome: the second genome of human body.” Protein Cell, 2010, DOI: 10.1007/s13238-010-0093-z
[2] Ley, R. E., Peterson, D. A. and Gordon, J. I. “Ecological and evolutionary forces shaping microbial diversity in the human intestine.” Cell, 2006, DOI:10.1016/j.cell.2006.02.017
[3] Wolf, K.J. and Lorenz, R.G. “Gut Microbiota and Obesity.” Curr Obes Rep, 2012, DOI: 10.1007/s12679-011-0001-8
[4] Fletcher G.F., Grundy S.M. and Hayman L.L. “Obesity: Impact on cardiovascular disease.” Circulation, 1998, DOI:10.1161/01.CIR.98.14.1472
[5] Tilg, H., and Kaser, A. “Gut microbiome, obesity, and metabolic dysfunction.” The Journal of Clinical Investigation, 2011, DOI:10.1172/JCI58109
[6] Ley, R., Turnbaugh, P., Klein, S., and Gordon, J. “Microbial ecology - human gut microbes associated with obesity.” Nature, 2006, DOI:10.1038/4441022a
[7] Catalan, V., Gomez-Ambrosi, J., Ramirez, B., Rotellar, F., Pastor, C. and Silva, C. “Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass”. Obes Surg, 2007, DOI:10.1007/s11695-008-9424-z
[8] Fessler, M.B., Rudel, L.L. and Brown, J.M. “Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome.”Curr Opin Lipidol, 2009, DOI: 10.1097/MOL.0b013e32832fa5c4
[9] Cani, P.D., Amar, J., Iglesias, M.A., Poggi, M., Knauf, C. and Bastelica, D. “Metabolic endotoxemia initiates obesity and insulin resistance.” Diabetes, 2007, DOI:10.2337/db06-1491
[10] Cani, P. D., Lambert, D. M., Muccioli, G. G., Delzenne, N. M., Possemiers, S., Van de Wiele, T. and Neyrinck, A. “Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability.” Gut, 2009, DOI:10.1136/gut.2008.165886
[11] D'Aversa, F., Tortora, A., Ianiro, G., Ponziani, F. R., Annicchiarico, B. E., and Gasbarrini, A. “Gut microbiota and metabolic syndrome.” Internal and Emergency Medicine,2013, DOI:10.1007/s11739-013-0916-z
[12] Bäckhed, F., Ding, H., Wang, T., Hooper, L.V.,Koh, G.Y., Nagy, A., Semenkovich, C.F. and Gordon, J.I. “The Gut Microbiota as an Environmental Factor That Regulates Fat Storage.” Proc. Natl. Acad. Sci. U. S. A., 2004, DOI:10.1073/pnas.0407076101
[13] Cani, P. D., Lecourt, E., Dewulf, E. M., Sohet, F. M., Pachikian, B. D., Naslain, D. and Delzenne, N. M. “Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal.” The American Journal of Clinical Nutrition, 2009, DOI:10.3945/ajcn.2009.28095
[14] Delzenne, N. M., Neyrinck, A. M., Bäckhed, F. and Cani, P. D. “Targeting gut microbiota in obesity: Effects of prebiotics and probiotics.” Nature Reviews. Endocrinology, 2011, DOI:10.1038/nrendo.2011.126
[15] Evans, D. F., Pye, G., Bramley, R., Clark, A. G., Dyson, T. J., & Hardcastle, J. D. “Measurement of gastrointestinal pH profiles in normal ambulant human subjects.” Gut, 1998, DOI:10.1136/gut.29.8.1035
[16] Hepburn, J.S., Eberhard, H.M., Ricketts, R. and Rieger, C.W. “Temperature of the gastrointestinal tract: the effect thereon of hot and cold foods and of physical therapeutic agents.” Arch Intern Med, 1933, DOI:10.1001/ archinte.1933.00160040109006.
[17] Ley, R.E., Bäckhed, F., Turnbaugh, P., Lozupone, C.A., Knight, R.D. and Gordon, J.I. “Obesity alters gut microbial ecology.” Proc. Natl Acad. Sci. USA, 2005, DOI: 10.1073/pnas.0504978102
[18] Backhed, F., Manchester, J. K., Semenkovich, C. F., and Gordon, J. I. “From the cover: Mechanisms underlying the resistance to diet-induced obesity in germ-free mice.” Proceedings of the National Academy of Sciences, 2007, DOI:10.1073/pnas.0605374104
No comments:
Post a Comment