Coronavirus Uses Spikes To Break Into Cells - Here’s How To Stop It
Coronaviruses are named after the crown or 'corona' that surrounds each virus particle, a crown of thorns made up of spike proteins. Those spikes interact with molecules on the surface of a cell so that the virus can invade its host.
This article is a not-too-technical round-up of the latest research on what we know about the viral parasite causing the ongoing COVID-19 (Coronavirus Disease 2019) pandemic, SARS-CoV-2, the key molecules involved in allowing it to invade human cells, and how scientists will exploit that information to stop the virus.
The ACE2 receptor
A virus can't enter at any old place on a cell's membrane as if it were bashing through the wall of a building, it has to go through a window. Cells are covered in a wide variety of surface molecules with functions that are initiated after they serve as receptors for other molecules. Receptors are the windows that viruses access.
Most Popular In: Science
Molecular interactions are somewhat esoteric, so from this point on I'll explain what happens by extending the analogy to a burglar breaking and entering a factory, where the cell's receptor is the lock and — in the case of coronaviruses — the viral spike protein is the lock pick. The burglar is actually a robot that wants to upload its blueprints to a computer connected to 3D printers that would then mass-produce copies of the burglar, which is how a virus replicates after fusing with a cell and unloading genetic instructions.
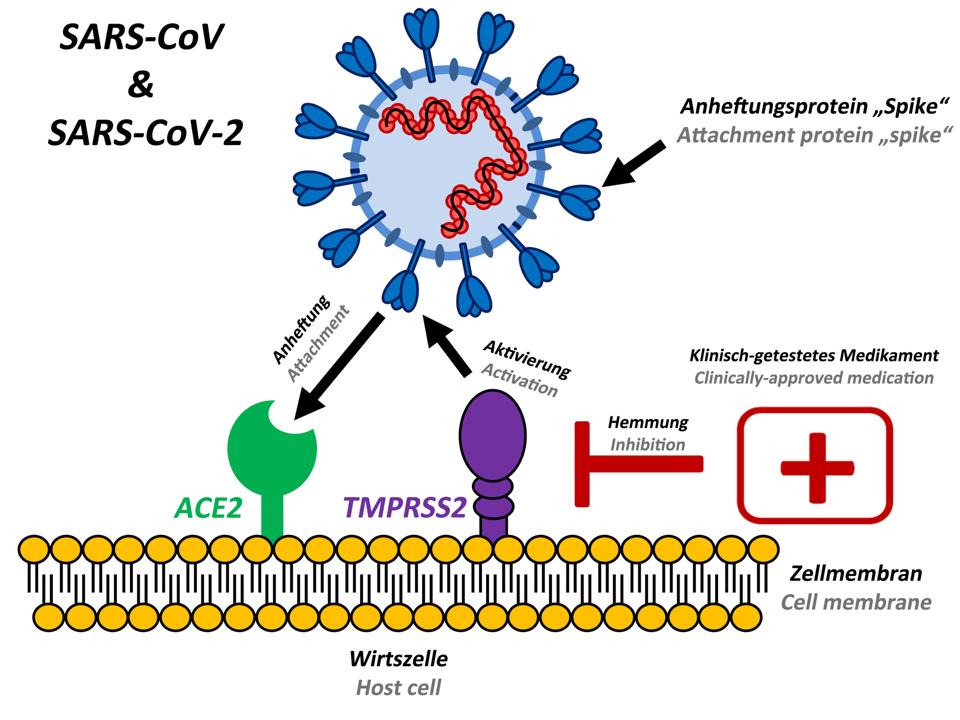
Which molecule does SARS-CoV-2 use to break into human cells? Following the 2003 SARS (Severe Acute Respiratory Syndrome) epidemic, immunologists at Harvard Medical School demonstrated that the virus responsible, SARS-CoV, uses its spike proteins to invade cells via ACE2 (Angiotensin-Converting Enzyme 2). This year, virologists at the Chinese Academy of Sciences in Wuhan then confirmed that SARS-CoV-2 attaches to the same receptor.
ACE2's functions include ultimately acting as a vasodilator that influences blood flow. It's located on cells all around the body but biomedical researchers at Shanghai Jiao Tong University showed that while the ACE2 receptor occurs in many organs, it's especially common on cells lining air sacs (pneumocytes) in the lungs, which is why infection can cause respiratory symptoms like pneumonia. Given its vital role around the human body, ACE2 may not be the best target for drugs.
The spike protein
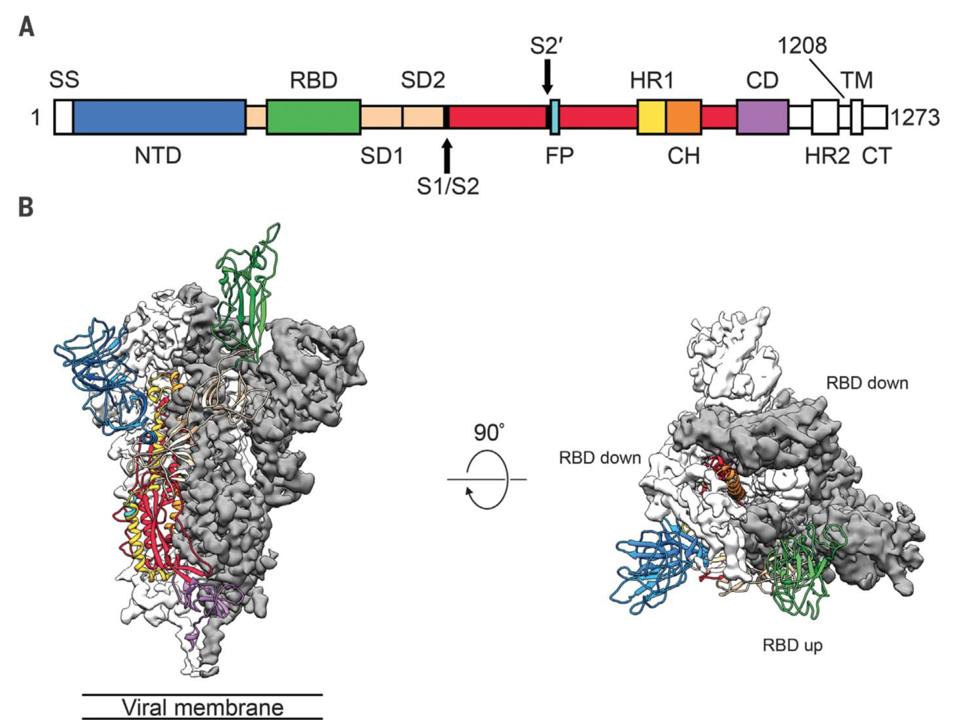
Each spike protein consists of three components that combine to form a 'trimer' structure with two parts or 'subunits', S1 and S2. You can think of the spike as a multistage rocket, with S1 being the boosters and S2 as a space shuttle: once attached to the ACE2 receptor, a spike sheds its S1 subunit and the remaining S2 part changes its shape or 'conformation' to enable the viral envelope to fuse with the outer membrane and drop the virus' genetic material inside the cell.
The shape-shifting nature of the spike protein was revealed for SARS-CoV as well as the coronavirus behind MERS (Middle East Respiratory Syndrome) in 2017, through cryogenic electron microscopy (cryo-EM) by biochemists at the University of Washington, Seattle. The same team has since reported that the spikes of both SARS-CoV and SARS-CoV-2 bind ACE2 to similar degrees, and that antibodies against SARS-CoV's spikes can block SARS-CoV-2 from entering cells.
Before the fusion of viral envelope and cell membrane, the spike protein has a different conformation. Using the cryo-EM technique, molecular biologists at the University of Texas at Austin determined the structure of the SARS-CoV-2 spike in its 'prefusion' shape, which provides a map of potential targets for vaccines, therapies, diagnostic tests or antiviral drugs. Those targets include the part that can recognize and attach to a cell's ACE2 receptor, the spike's receptor-binding domain, although the biologists found that antibodies matching SARS-CoV's domain didn't bind SARS-CoV-2.
Do antibodies against the spike of SARS-CoV protect against SARS-CoV-2, or not? There could be any number of reasons for the discrepancy in results between the two research groups. The Washington team used 'polyclonal' antibodies from immune cells that would recognize various parts on the viral spike protein, for example, whereas the Texas group used 'monoclonal' antibodies specific to the spike's receptor-binding domain. At present it's unclear whether an antibody therapy based on the coronavirus that causes SARS would work for COVID-19.
Understanding how the spike protein and ACE2 receptor interact is critical to planning an effective approach to stopping SARS-CoV-2 infections, which is why it's interesting to note that structural biologists at Westlake University in Hangzhou, China, showed that one ACE2 receptor actually accommodates two spike proteins. Meanwhile, researchers at New York Blood Centre found that a spike's receptor-binding domain alone can attach to ACE2, which suggests parts of a spike might work as a 'viral attachment inhibitor' that blocks live viruses from entering cells, like leaving a broken key stuck inside a lock.
The TMPRSS2 protease
The interaction between spike proteins and the ACE2 receptor is clearly more complicated than a simple lock-and-key relationship. Many more molecules may be involved in the process allowing SARS-CoV-2 to invade cells. At the moment, we know of at least one other key player: TMPRSS2 (transmembrane serine protease 2). Adding it to the burglary analogy becomes a bit forced, but you can think of TMPRSS2 as an inside man working for the factory that the burglar wants to turn into a robot-manufacturing plant: TMPRSS2 meets the burglar outside the building to prepare or 'prime' the lock pick (spike) so it will properly fit the factory's locks.
TMPRSS2 is a protease enzyme, a type of protein that cuts other proteins, and is another potential target for drugs. Biologists at the German Primate Centre in Göttingen found that SARS-CoV-2 depends on TMPRSS2 protease to invade cells and more importantly from a therapeutic perspective, showed that a protease inhibitor previously approved for clinical use, camostat mesylate, can block the virus from entering cells. In the analogy, the inhibitor is a security guard who intercepts the inside man before they prepare the burglar's lock pick.
The Göttingen team also found that antibodies isolated from SARS patients, which surround and neutralize the SARS-CoV spike protein at high efficiency, can also 'cross-neutralize' SARS-CoV-2's spikes to a moderate extent. This would be like the factory learning from a prior break-in and changing the locks, and suggests that a SARS vaccine that prompts the body to produce antibodies against SARS-CoV could partially protect against SARS-CoV-2 and possibly prevent COVID-19. This result seems to support the University of Washington's study, but could also be explained by antibody neutralization of other parts ('epitopes') of antigens from SARS-CoV-2, not the receptor-binding domain of its spike protein.
All the studies I've described above were done in lab-grown cells, not humans or even animal models. Due to the phenomenal speed at which COVID-19 is spreading, however, clinical trials are progressing at a rapid pace in order to determine which approaches and treatments might work, and are safe. Armed with that knowledge, it's only a matter of time before scientists find a way to fight back against SARS-CoV-2.
No comments:
Post a Comment